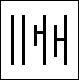Colour change Paraiba tourmaline from Brazil
Keywords: Paraiba tourmaline, Brazil, colour change
In just a few decades Paraiba tourmalines have developed into one of the most valuable and sought-after gems in the world. Of particular interest are specimens from the legendary Batalha mine near Sao Jose de Batalha in the Brazilian state Paraiba were these gemstones were first mined. However, fine specimens from Batalha are rare and production in Brazil is sporadic and focused on melée and matrix stones obtained from the overburden.
Peak production with about ninety percent of the tourmalines recovered from the site occurred between 1989 and 1992 (Cook, 1993). Recently a number of Paraiba tourmalines ranging between 0.75 ct and 1.75 ct. were submitted to the DSEF German Gem Lab. According to the owners they were kept on stock and originally purchased in the early 1990ies.
When viewed table up some of these stones showed a colour change from violetish-blue to blue-violet in daylight to a greyish-green-blue to greyish blue-green in incandescent light, respectively. A 0.81 ct. specimen is shown in Figure 1. It measures approx. 7.30 x 5.24 x 3.31 mm and changes from blue-violet to green blue.

Colour change in cuprian elbaite tourmaline has been reported for a small number of samples from Nigeria (Smith et al., 2001) and Mozambique (Wentzell 2004, Wentzell et al. 2005, Laurs et al. 2008). However, to our best knowledge this is the first report on colour change Paraiba tourmalines from Brazil. Chemical analyses were consistent with elbaite tourmaline. The elevated copper concentrations are comparable to those found in Brazilian specimens and the low Ga contents are typical for samples from the Batalha mine. Most cuprian tourmalines from Rio Grande do Norte show higher Ga contents.
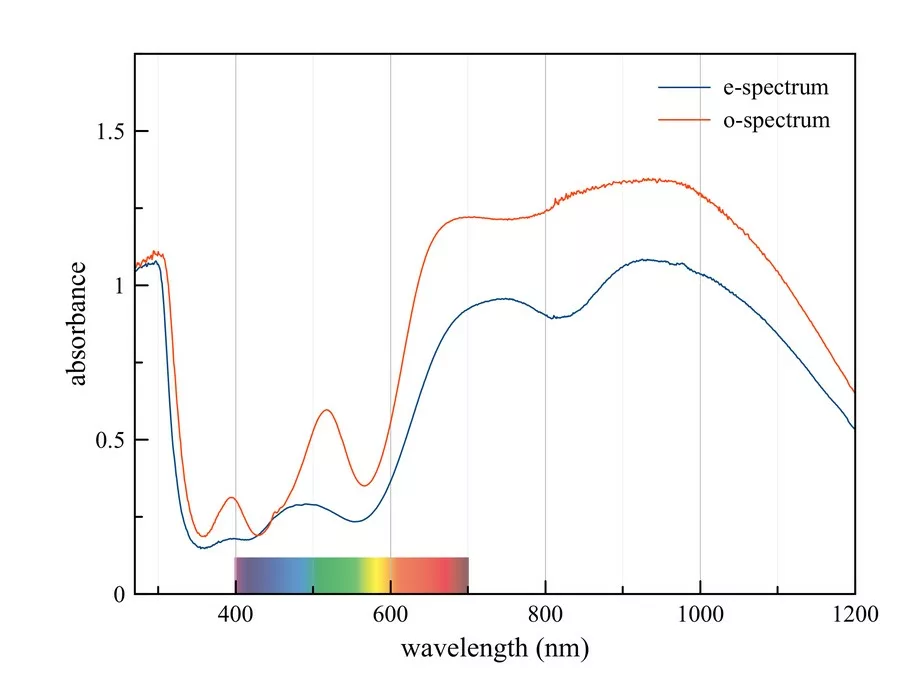
Polarized absorption spectra of the sample shown in Figure 1 showed broad absorption bands at around 395 nm, 520 nm as well as at 690 and 910 nm in the o spectrum and at 395 nm, 485 nm and 730 and 940 nm in the e spectrum (Figure 2). The distinct bands at 395 and 520 nm can be attributed to trivalent manganese typically found in unheated Paraiba tourmalines and the two bands in the red and infrared part of the electromagnetic spectrum are due to divalent copper. The absorption band of Mn3+ at 520 nm forms two transmission windows, one in the blue-violet and one in the yellow-green part of the visible spectrum, which are responsible for the color change.
The question arises as to why no colour change Paraiba tourmalines from Brazil have been observed so far. The colour of natural coloured, unheated tourmalines is far less attractive than the coveted “neon colors” that made these tourmalines so well known. From the absorption spectra it is obvious that the stones examined are all suitable for heat treatment and therefore it is very likely that in the past most of the stones had been heat treated before they were released on the market.
References:
- Cook, B., 2013. The Paraíba promise. Rapaport Magazine 36(10), 130.
- Laurs, B.M. et. al, 2008. Copper-bearing (Paraiba-type) tourmaline from Mozambique. Gems & Gemology 44(1), 4-30.
- Smith, C.P., Bosshart, G., Schwarz, D., 2001. Nigeria as a new source of copper-manganese-bearing tourmaline. . Gems & Gemology 37(3), 239-240.
- Wentzell, C., 2004. Copper-bearing color change tourmaline from Mozambique. Gems & Gemology 40(3) 250-251.
- Wentzell, C., Fritz, E., Muhlmeister, S., 2005. More on copper-bearing color change tourmaline from Mozambique. Gems & Gemology 41(2), 173-175.