A study of nickel-bearing type Ia diamonds
By M. Mintrone, L. Phan and J-P. Chalain
We present a detailed FTIR and PL spectroscopic study (i.e. at 298 and 77°K) of a large number of colorless (i.e. color D to G) type Ia diamonds (i.e. >200), showing the presence of the 787 nm peak (1.575 eV). We detected this center in at least ca. 40% of more than 8000 analyzed diamonds. All these diamonds are type Ia, as previously described by Vohra et al., (1989). This peak is always associated with Ni-related centers (e.g. 496.7 nm center (S3)), and hence thought to be related to nickel-nitrogen defects (Dobrinets et al., 2013 and reference therein).
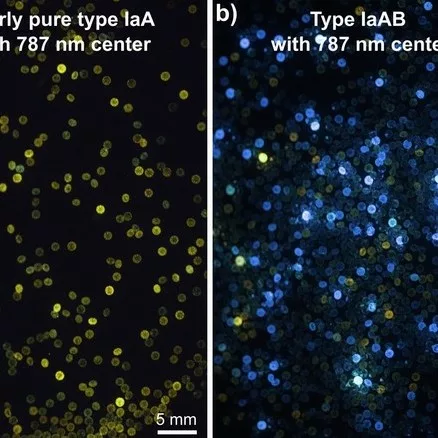
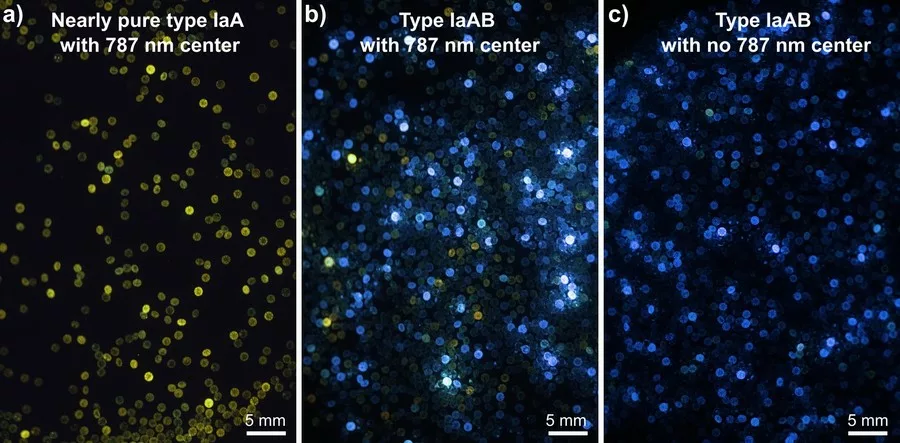
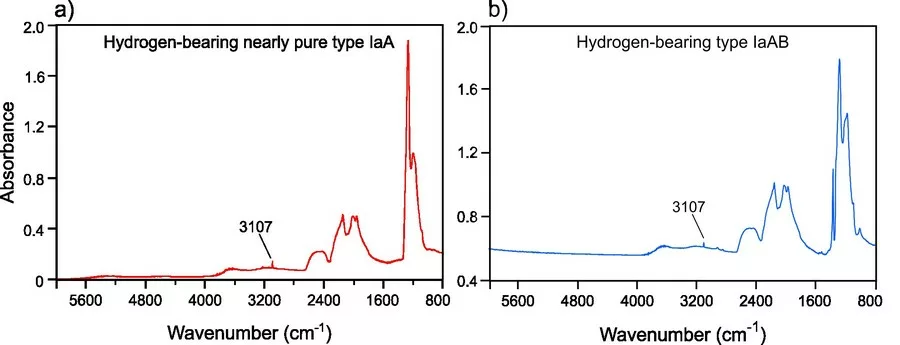
Our preliminary results show that most of these diamonds are type IaAB showing no to strong blue fluorescence, while a non-negligible proportion of nearly pure type IaA diamonds (i.e. 5-10 %) show no to strong yellow fluorescence (Fig. 1a, b). Moreover, at least ca. 90% of these diamonds (i.e. type IaA and IaAB) show the characteristic infrared absorption peak at 3107 cm-1 (VN3 H) (see Fig. 2), corresponding to a well-known hydrogen center in natural type Ia diamond (e.g. Fritsch and Scarratt, 1989; Goss et al., 2014). Photoluminescence spectra in the visible and near-infrared regions always show the 787 nm center associated with nickel-nitrogen defects (see Fig. 3). The 694, 701, and 794 nm centers were described in untreated natural and synthetic diamonds and are thought to be related to the presence of nickel (Chalain 2003; Dobrinets et al., 2013 and reference therein). The 709 nm center may correspond to the 711 nm Ni-related center observed by Yelisseyev and Kanda (2007), which coupled with the presence of the 787 and 794 nm centers constitutes a reliable indicator of untreated natural diamonds (Dobrinets et al., 2013).
The presence of nickel in diamonds is thought to facilitate the aggregation of nitrogen, which leads to the combination of single dispersed nitrogen atoms to more complex aggregates such as A and B centers. The catalytic role of nickel may be due to the higher mobility of nickel-nitrogen complexes compared to the “pure” nitrogen defects (Dobrinets et al., 2013). Hence, most of the natural nickel-containing diamonds formed and stored at the Earth’s mantle temperature during geological timescales (i.e. at T> ca. 1000°C for 103 -109 years, e.g. Stachel and Harris, 2009) should easily reach the formation of B center. Indeed, only ca. 5-10% of our studied diamonds containing nickel are nearly pure nitrogen-rich (i.e. > ca. 500 ppm of N) type IaA diamonds (i.e. with no or only a few ppm of B center, and no platelets) with no detectable N3 defect. Nevertheless, these diamonds show a VN3 H center, testifying of an advanced nitrogen-hydrogen aggregation state (e.g. Goss et al., 2014). This result may be consistent with recent studies suggesting that hydrogen can potentially modify the nitrogen aggregation route (e.g. Wood 2021 and reference therein). However, it is important to note that the formation of the VN3 H center and its influence on other aggregated defects is still poorly constrained and requires more study. As a consequence, our study aims at better understanding the role of hydrogen and nickel in natural diamonds and their influence on the aggregation process of nitrogen.
References:
- Chalain J. P. (2003) A natural yellow diamond with nickelrelated optical centers. G&G 39, 325-356.
- Dobrinets I.A., Vins V.G., Zaitsev A.M. (2013) HPHT-Treated Diamonds: Diamonds Forever. Springer, Heidelberg, Germany.
- Fritsch E. and Scarratt K. V. G. (1989) Optical Properties of Some Natural Diamonds with High Hydrogen Content, Proc. SPIE 1146, Diamond Optics, https://doi. org/10.1117/12.962079.
- Goss J.P., Briddon P.R., Hill V., Jones R., Rayson M.J. (2014) Identification of the structure of the 3107 cm−1 H-related defect in diamond. J. Phys.: Condens. Matter 26 145801.
- Stachel T. and Harris J. W. (2009) Formation of diamond in the Earth’s Mantle. J. Phys.: Condens. Matter 21 364206. DOI 10.1088/0953-8984/21/36/364206
- Vohra Y. K., Vanderborgh C. A., Desgreniers S., Ruoff A.L. (1989) Near-infrared photoluminescence bands in diamond. Phys. Rev. B, 39 5464-5467.
- Wood J. O. (2021) An elusive impurity: studying hydrogen in natural diamonds. Ph.D. thesis, University of Bristol. 218 pages.
- Yelisseyev A. P. and Kanda H. (2007) Optical centers related to 3D transitional metals in diamond. New Diam. Front. Carbon Technol. 17(3), 127-178.