Treated jadeite-jade: Unusually bright fancy colours
Introduction
Jadeite-jade is a precious ornamental stone that has been valued as the topmost decorative stone and has been used in jewelry, carvings, and other ornamental objects by many cultures throughout history, particularly in China and other parts of East Asia. The colour origin and treatment of jades are important factors that may impact the price of the stone. Natural untreated jadeite is called A-type in the Asian trade. The most common enhancement for jadeite-jade is polymer impregnation, classified as B-type, and dying, called C-type. Recently, the GIT-Gem Testing Laboratory (GIT-GTL) received six oval cabochons for identification which showed unusual bright fancy colours. Their striking fancy colour gave us immediately the impression that these samples were not common-coloured stones of natural origin. Even though jadeite-jades of such colour had previously been seen in the market and mentioned by Mock and Hughes (2022), these samples were extraordinarily bright and colourful that needed an in-depth investigation.

Materials and methods
The studied six oval cabochons (Figure 1) possess unusual bright body colours as purple (FJBC001, 7.63 ct), purplish red (FJBC002, 83.42 ct), dark purplish red (FJBC003, 74.06 ct), blue-green (FJBC004, 76.25 ct), bluish green (FJBC005, 95.70 ct) and yellow (FJBC006, 68.22 ct). Such bright and fancy colours are not common for jades seen in the market. All samples were examined using standard gemological tools, and advanced equipment; a PerkinElmer Lambda 1050 UV-VIS-NIR spectrophotometer measured in UV-Vis (380-730 nm) range, a Thermo-Nicolet iS50 FTIR spectrometer in the mid-IR (400-4900 cm-1) and near-infrared (3700- 6500 cm-1) regions, a Renishaw inVia Raman spectrometer with a 532 nm Nd:YAG laser excitation and EDXRF Eagle III for chemical analysis.
Gemological Features
All the stones showed similar RI of 1.65-1.66 (spot reading) and SG of 3.26 -3.31 that are typical for jadeite. Furthermore, all samples exhibited strong bluish white reaction under LWUV and a weaker bluish white reaction under SWUV lamps. Such a fluorescent appearance is often found in polymer-impregnated jade.
Microscopic observations
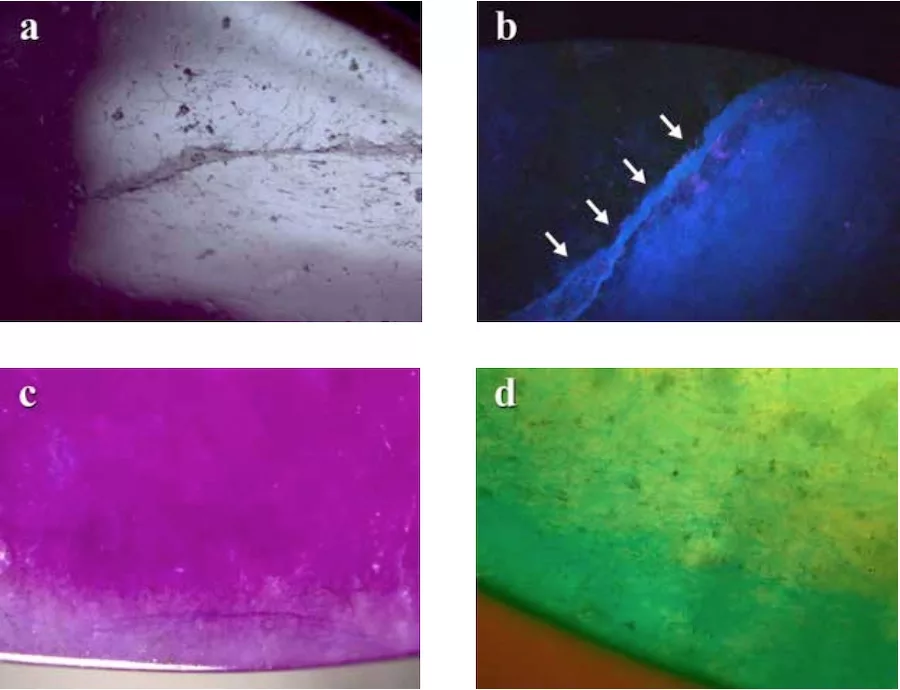
Visually, these samples appeared to have even colour distribution and a smooth, polished surface texture. However, when observed under higher magnification, the purplish red sample (FJBC002) revealed distinctly large and small fractures and grain boundaries clearly filled with a material reaching the surface (Figure 2a). The yellow sample (FJBC006) similarly showed a large open fracture filled with a material exhibiting a strong blue reaction under LWUV (Figure 2b). Thus, the filling material in all samples was likely a polymer as it showed strong blue fluorescence under LWUV. Using fiber-optic light, dyed-colour concentrations along fractures and grain boundaries were evident in almost all samples (see Figure 2c,d), but less obvious in the yellow sample.
Spectroscopic Features Raman-spectroscopy The identity of the stones was also confirmed by Raman spectroscopy. The Raman spectra of all samples showed dominant peaks at approximately 203, 370, 430, 522, 570, 695, 982, 1040 cm-1 (Raman shift) which perfectly match with the jadeite reference spectrum from the RRUFF database. The presence of polymer peaks was also detected around 2500-3000 cm-1.
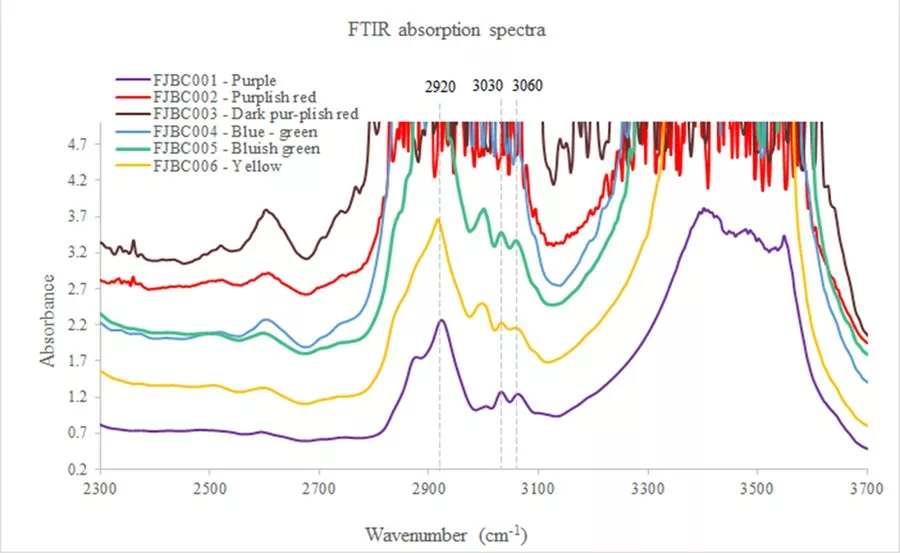
Mid-Infrared Absorption Spectra The mid-infrared spectra of the samples revealed characteristic absorption bands of polymers at approximately 2920, 3030 and 3060 cm–1 that match with a family of phthalates (Fritsch et al., 1992) (Figure 3). In the near-infrared spectra, all samples also showed additional bands of jadeite approximately 6200-5500 cm-1 and other features at 4050, 4530, 4612, 4665, 4880, 4970, and 5230 cm-1 that were identified as plastic (variety of organic polymer), which is likely a mixture of different polyacrylates (Hurwit, 1989). Semi-quantitative chemical analyses Semi-quantitative chemical analyses of all samples by EDXRF showed distinct concentrations of Na2 O (12.66- 15.02%), Al2 O3 (23.86-25.89%), SiO2 (59.07-60.81%) with minor CaO (0.16-1.26%), and traces of V2 O3 (0.01-0.14%), Cr2 O3 (<0.01-0.06%), Fe2 O3 (0.12-1.07%), TiO2 (<0.01-0.19%) and MnO (0.01-0.04%). These analyses are well in accordance with the expected composition of jadeite. Almost all samples contain relatively high iron contents.
UV-Visible Absorption Spectra
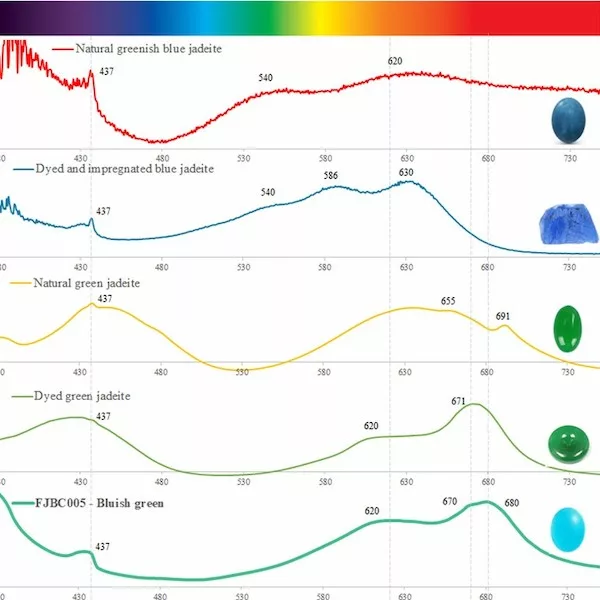
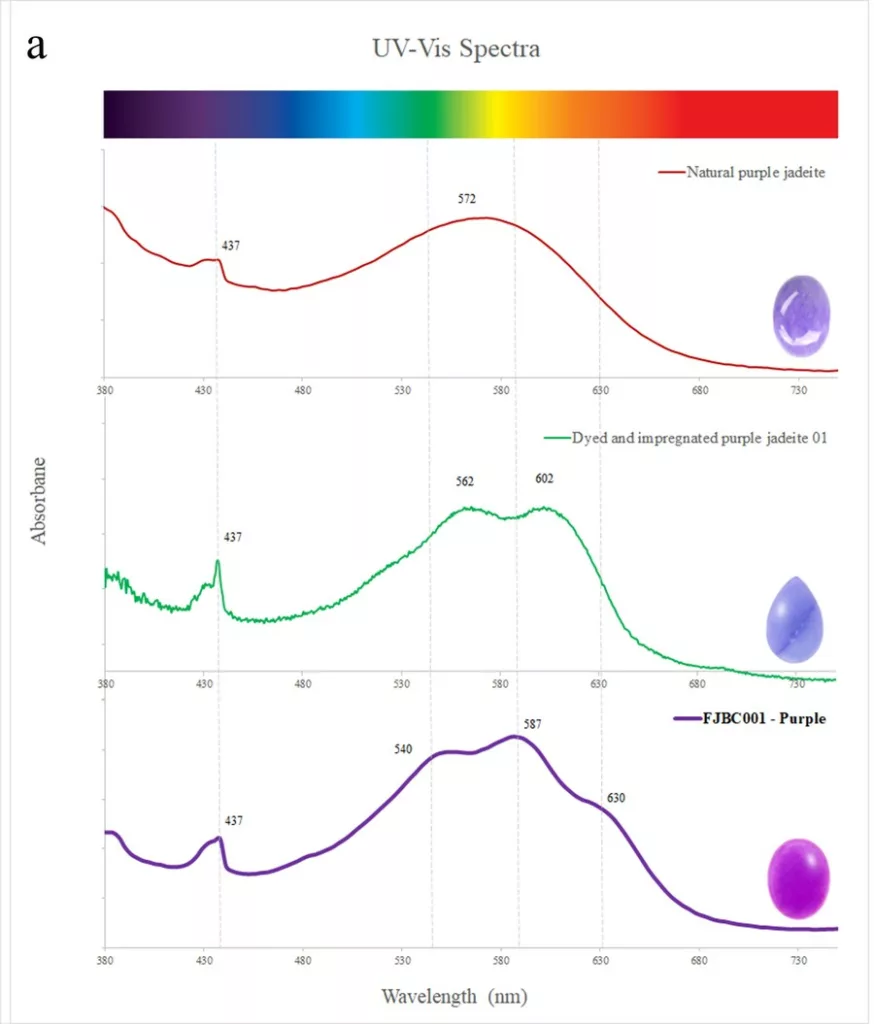
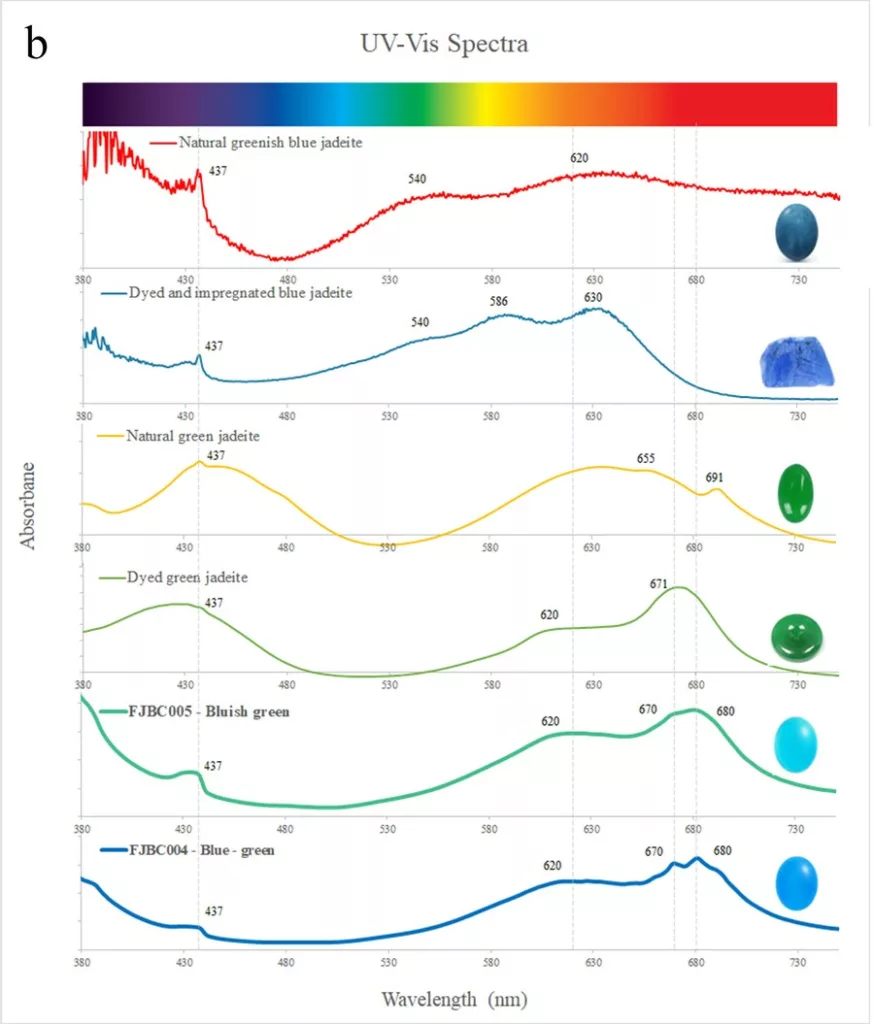
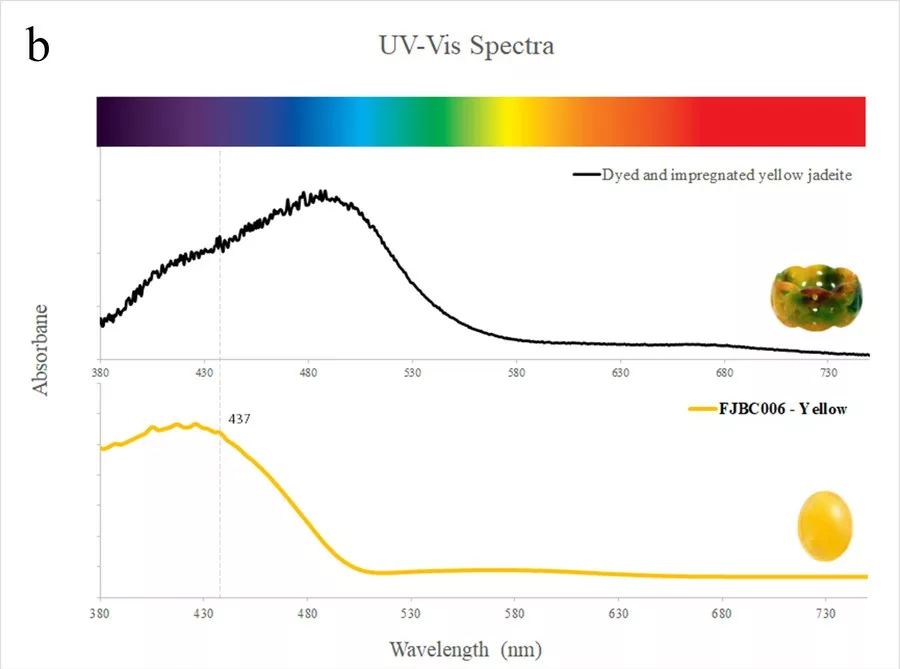
Nearly all samples showed a small absorption peak at 437 nm due to Fe3+. This peak is commonly found in jadeite of various colours (Koivula, 1982) and related to the relatively high iron contents of the investigated samples. The dyed purple sample (FJBC001, see Fig. 4a) displayed triple broad bands at about 540, 587 and 630 nm, likely related to the purple dye. The spectrum of this dyed sample is somewhat similar to a reference spectrum of a dyed and impregnated purplish jadeite (double broad bands near 562 and 602 nm, green line in Fig. 4a), but fairly different from the spectrum of a purple jadeite of natural colour (a large broad band near 572 nm due to Mn, red line in Fig. 4a) from our reference collection. The spectra of the dyed samples of blue-green (FJBC004) and bluish green (FJBC005) colours (Figure 4b) exhibited quite similar absorption features, a small Fe3+ related peak at 437 nm and strong broad bands at about 620, 670 and 680 nm. These broad bands were probably caused by the blue-green dye. These absorption features are similar to a dyed green jadeite from our reference collection except the dyed green reference sample has also a strong absorption band in the blue-violet, thus shifting the transmission window to the green. The absorption features of the studied blue-green and bluish green samples, however, are quite different to green jadeite of natural colour (typical Cr3+ related bands at about 437, 655 nm and a sharp peak at 691nm, yellow line in Fig. 4b). Our -blue-green and -bluish green dyed samples also show different absorption bands compared to dyed and impregnated blue jadeite (greenish blue line in Fig. 4b) and greenish blue jadeite of natural colour from our reference collection (red line in Fig. 4b).
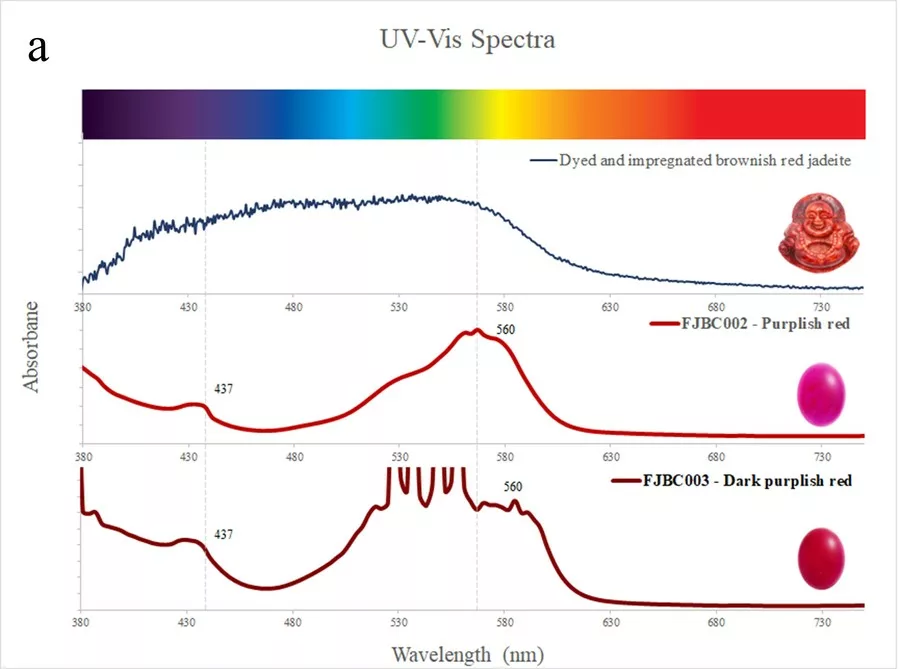
The spectra of dyed samples of purplish red (FJBC002, red line in Fig. 5a) and dark purplish red (FJBC003, dark red line in Fig. 5a) colours display fairly similar absorption features, i.e. a small Fe3+ related peak at 437 nm and a strong broadband between 530 and 580 nm, probably caused by the red dye. The spectra of these two samples are however different from the spectrum of a dyed and impregConclusion Standards gem properties and the strong bluish white fluorescence reaction under LWUV clearly indicate that these samples are jadeite-jades that have been polymer-impregnated. High magnification revealed fluorescent polymer fissure filling and colour concentrations along fractures and grain boundaries strongly indicating a dye treatment. The identity of these samples as jadeite was also confirmed by Raman micro-spectroscopy. The presence of a polymer-impregnation was clearly detected by FTIR spectroscopy. The UV-Vis absorption spectra of all samples showed a small Fe3+-related peak at 437 nm and various broad absorption bands related to their specific colour and dye. The spectra showed some similarities with dyed samples from our reference collection, but were distinctly different from jadeite of natural colour (purple, green, green-blue) from our reference collection. Based on our analyses of these bright fancy colour stones, these samples are identified as dyed nated jadeite of brownish red colour from our reference collection (blue line in Fig. 5a). The spectrum of the dyed sample of yellow colour (FJBC006, yellow line in Fig. 5b) reveals a strong absorption band below ~480 nm towards the UV region, likely related to yellow dye, which matches somehow the spectrum of the dyed yellow sample of our reference collection.
Conclusion
Standards gem properties and the strong bluish white fluorescence reaction under LWUV clearly indicate that these samples are jadeite-jades that have been polymer-impregnated. High magnification revealed fluorescent polymer fissure filling and colour concentrations along fractures and grain boundaries strongly indicating a dye treatment. The identity of these samples as jadeite was also confirmed by Raman micro-spectroscopy. The presence of a polymer-impregnation was clearly detected by FTIR spectroscopy. The UV-Vis absorption spectra of all samples showed a small Fe3+-related peak at 437 nm and various broad absorption bands related to their specific colour and dye. The spectra showed some similarities with dyed samples from our reference collection, but were distinctly different from jadeite of natural colour (purple, green, green-blue) from our reference collection. Based on our analyses of these bright fancy colour stones, these samples are identified as dyed and impregnated (B+C type) jadeite-jades of very unusual and bright “fancy” colours.
References:
- Fritsch, E., Wu, S.T.T., Moses, T., McClure, S.F., Moon, M., 1992. Identification of bleached and polymer- impregnated jadeite. Gems & Gemology, 28(3), 176-187
- Hurwit, K., 1989. Gem trade lab notes: Impregnated jadeite jade. Gems & Gemology, 25(4), 239-240
- Koivula, J.I., 1982. Some observations on the treatment of lavender jadeite. Gems & Gemology, 18(1), 70-85
- Mock, D.W.K., Hughes, R.W., 2022, Jadeite jade and its identification, In Jade: A Gemologist’s Guide by R.W. Hughes, RWH Publishing, Bangkok, 533pp.