Explanation on Yellow, Green and Brown Coloration Series of Basalt Related Bangkaja Sapphires
Keywords: Yellow sapphire, brown sapphire, green sapphire, Bangkaja, Chanthaburi, band model, UV-Vis-Nir absorption, FTIR
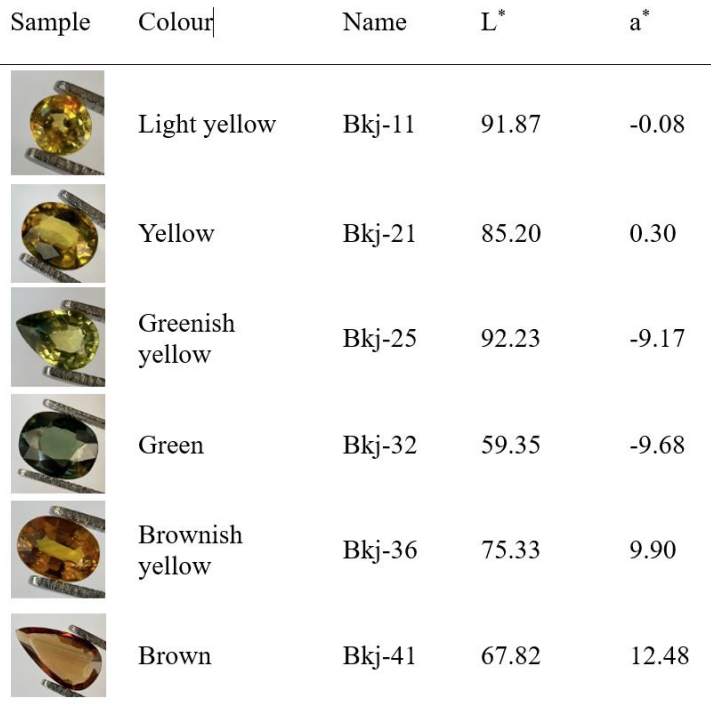
The basalt related Bangkaja sapphires, excluding the black star ones, commonly occur in three coloration series, i.e., the yellow, the green and the brown. Two representative samples of each colour series, altogether six, were selected from our Bangkaja sapphire collection (43 stones); those six samples, i.e., light yellow and yellow, greenish yellow and green, and brownish yellow and brown represent the yellow, the green and the brown colour series, respectively. They have been studied to explain the true causes of their colour variations by using band models. Trace element chemistry, UV-Vis-NIR (both absorption and excitation), and FTIR spectra of the samples were recorded. Besides, their L*a*b* colour notations were measured using UV-Vis reflectance spectroscopy (Table 1).
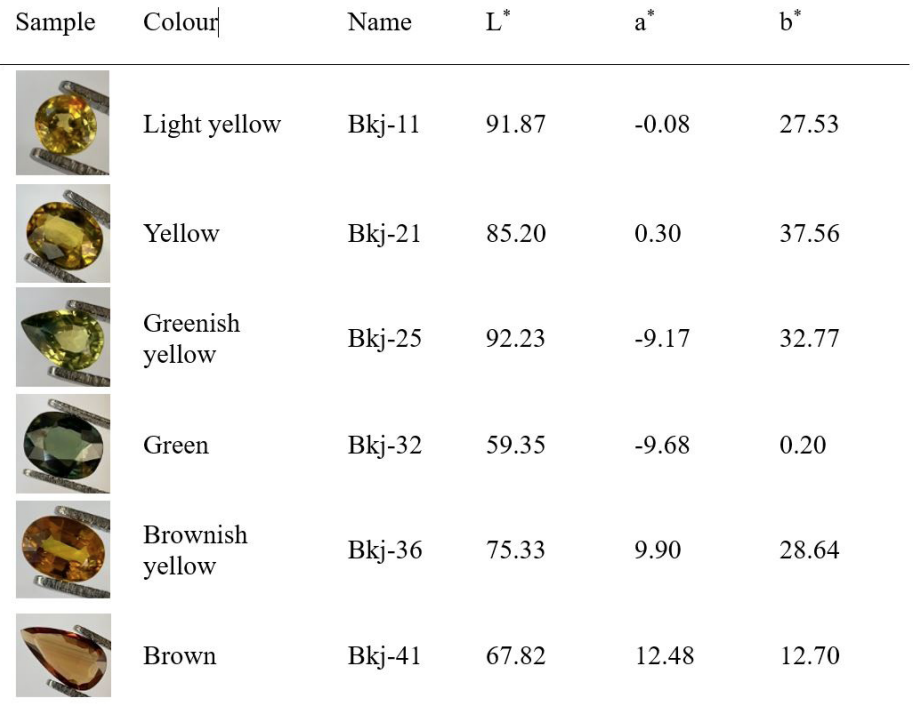
The results confirm the pure yellow component is caused by Fe3+ with increasing intensity to the bright brown coloration so called the Mekong whisky colour, the most preferable yellow sapphires from Bangkaja, Chanthaburi, Thailand, which also commands high pricing in the market among Thai people. This colour series shows UV-Vis absorptions at 377 nm, 388 nm and 450 nm similar to their polychromatic UV-Vis excitation spectra confirming that the samples are natural without beryllium heat treatment (Monarumit et al., 2020 and 2023). The more intense brown stones also show pronounced absorption at 450 nm which is also designated for the Fe3+/Fe3+ forbidden state (Häger, 2001). The tinted green samples, i.e., greenish yellow gradually ranges to green stones tend to have an increasing of Ti content. The three series have proven that the green is caused by gradually adding the blue component to the yellow which finally turns to blue with elevated Ti together with Fe content. Though this is rather commonly understandable, however, when the blue component has been added to the yellow, and the green (also blue-green till blue) varieties visually appear, the 580 nm and 710 nm absorptions present with a remarkably additional broad absorption at around 890 nm. Besides, their FTIR absorptions display the structural Ti-OH peak at 3309 cm-1 (Phlayrahan et al., 2018a, 2018b and 2019) while the greenish tint increases, finally the whole 3309 cm-1 series (3309 cm-1, 3232 cm-1 and 3184 cm-1) appears in the blue Bangkaja stones. It is thus revealed that the 580 nm, 710 nm and 890 nm absorptions are related to Ti. The increasing 890 nm absorption also normally indicates green/blue sapphires of basalt hosted known by most gem scientists (e.g., Palke et al., 2019). Hence, it is time to explain and understand, why there is no 3309 cm-1 absorption and its series in yellow/brown sapphires whereas those with blue tint do; this is because the Fe3+ and Fe3+/Fe3+ pairs provide balance substitution of Al3+ and 2Al3+ in the adjacent octahedral sites. Indeed, the Fe3+/ Ti4+ pairs proposed by our previous work using the XANES technique (e.g., Wongrawang et al., 2016; Mogmued, et al., 2017), are responsible for the blue component which is increasingly added to Bangkaja yellow sapphires resulting in greenish yellow and green stones. Therefore, an OH- is required to balance the one-more excess positive charge of octahedral structural units which contain the Fe3+/Ti4+ pair (7+) instead of Fe2+/Ti4+ (6+) as the IVCT concept previously claimed and widely followed (e.g., Townsend, 1968; Nassau, 1978; Burns, 1981; Fritsch and Rossman, 1988).
The band models of yellow, green, and brown colour series of Bangkaja sapphires have been proposed; the yellow component gradually ranges to the intense-brownish yellow to brown colorations due to Fe3+ and Fe3+/Fe3+ states in the yellow sapphire band gap (Figure 1a and c). Besides, the Fe3+/Ti4+ mixed acceptor states are additionally present in the green colour series (Figure 1b).
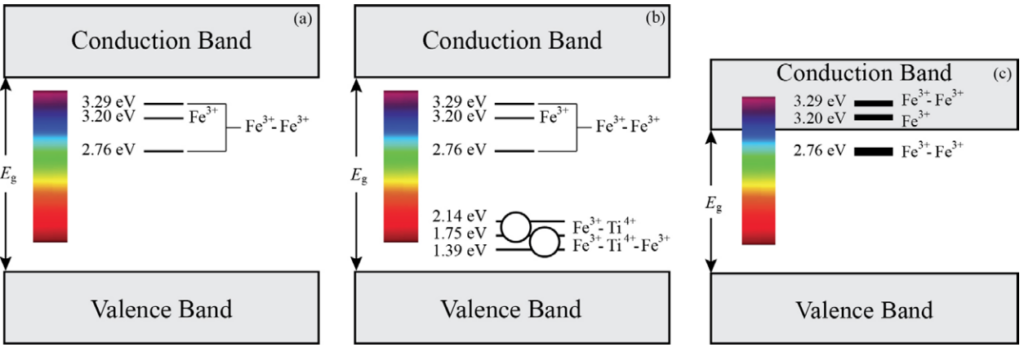
As the result of no-charge difference mentioned, the yellow as well as the brown sapphires never show the 3309 cm-1 FTIR absorption (structural OH) unless the blue component which is caused by the Fe3+/Ti4+ mixed acceptor pair existing in the sapphire structure, resulting in the green coloration, and eventually the blue one. In the unheated stones, the molecular water would normally appear as a broad FTIR absorption at around 3400 cm-1. The green coloration series of Bangkaja sapphires is caused by addition of a blue component (Fe3+/Ti4+ pair) to the yellow. Hence, the OH- is required only in greenish, green, and blue stones from Bangkaja. This explanation can also be implied to yellow, green (till blue), and brown sapphires occurring elsewhere.
References:
- Burns, RG., 1981. Intervalence transitions in mixed valence minerals of iron and titanium, Ann. Rev. Earth Planet. Sci. 9: 345-83
- Fritsch, E., Rossman, G.R., 1988. An update on color in gems. Part 2: Colors involving multiple atoms and color centers. Gems & Gemology, 24, 3-15
- Häger, T., 2001. Proc. Int. Workshop on Material Characterization by Solid State Spectroscopy: The Minerals of Vietnam, 24-37
- Mogmued, J., Monarumit, N., Won-In, K., Satitkune, S., 2017. Spectroscopic properties for identifying sapphire samples from Ban Bo Kaew, Phrae Province, Thailand, Journal of Physics Conference Series, 901, 012075
- Monarumit, N., Lhuaamporn, T., Sakkaravej, S., Wathanakul, P., Wongkokua, W., 2020. The color center of beryllium-treated yellow sapphires, Journal of Physics Communications, 4, 105018
- Monarumit, N., Lhuaamporn, T., Wathanakul, P., Saiyasombat, C., Wongkokua, W., 2023. The acceptor-donor pair recombination of beryllium-treated sapphires, Radiation Physics and Chemistry, 206, 110756
- Nassau, K., 1978. The origins of colour in minerals, The American Mineralogist, 63, 219-229
- Palke, A.C., Saeseaw, S., Renfro, N.D., Sun, Z., McClure, S.F., 2019. Geographic origin determination of blue sapphire, Gems & Gemology, 55(4), 536-579
- Phlayrahan, A., Monarumit, N., Satitkune, S., Wathanakul, P., 2018a. Role of Ti content on the occurrence of the 3309 cm-1 peak in FTIR absorption spectra of ruby samples. Journal of Applied Spectroscopy, 85, 385-390
- Phlayrahan, A., Monarumit, N., Boonmee, C., Satitkune, S., Wathanakul, P., 2018b. Fe oxidation state in heat-treated basaltic blue sapphire samples and its implication to the 3309 cm-1-series peaks in infrared absorption spectra, Journal of Physics: Conference Series, 1144, 012057
- Phlayraharn, A., Monarumit, N., Lhuaamporn, T., Satitkune, S., Wathanakul, P., 2019. Spectroscopic investigation revealing the heating experiment of blue sapphire samples. Journal of Applied Spectroscopy, 86, 810-816
- Townsend, MG., 1968. Visible charge transfer band in blue sapphire, Solid State Communications, 6, 81-83
- Wongrawang, P., Monarumit, N., Thammajak, N., Wathanakul, P., Wongkokua, W., 2016. Oxidation states of Fe and Ti in blue sapphire, Materials Research Express, 3, 026201
Acknowledgements:
The Gem and Jewelry Institute of Thailand (GIT), Department of Physics and Department of Earth Sciences, Faculty of Science, Kasetsart University are thanked for the research facilities.