Baltic amber and its inclusions: an insight into the origin and nature of the trapped material
Keywords: Baltic amber, inclusions, paleobiology
Amber is a fossilised resin from ancient trees (coniferous and deciduous), which underwent the process of fossilisation in various epochs and depositional environments. Until the past decade, it was thought to be very rare, but new discoveries have shown that it is more abundant in terms of both geographical coverage and presence through time than was previously thought. (Seyfullah and Schmidt, 2015). For a long time, amber has been used as a source of paleobiological information as it frequently contains well preserved elements of past ecosystems (Antoine et al., 2006; Schmidt et al., 2006, 2010; Girard et al., 2009). Fossil inclusions in amber receive a great deal of attention in the study of paleoecosystems and evolutionary history (e.g. Briggs, 2018; Ross, 2021; Sadowski et al., 2021) and represent a critical window to the evolution of terrestrial environments across the Mesozoic–Cenozoic transition (e.g. Martı́nez-Delclòs et al., 2004; Penney, 2010; Sadowski et al., 2021). Fossils (also known as inclusions) in amber often have exquisite, three-dimensional preservation, retaining fine surface and structural details, and are frequently preserved at least roughly in a position that they would have had in life, and before much decay has set in (Seyfullah and Schmidt, 2015). ‘Exceptionally preserved’ fossils in amber may owe their high quality to the biocidal properties of resin as well as its role as a ‘conservation trap’ (Briggs, 2003). Resin can protect its inclusions from the physical and biological processes that ordinarily destroy non-biomineralized tissues in terrestrial environments (Jiang et al., 2022). Amber has also been a source for investigations into the potential preservation of ancient DNA (Cano et al., 1994; Greenblatt et al., 1999), with controversial results. Resin does not just trap organisms but amber can also preserve vapour phases of various compositions and attempts have been made to analyse air and water bubbles contained in amber (Berner and Landis, 1988; Cerling, 1989) but the preservation of an original signal is still debated. They may represent ancient air trapped at the time the original resin was exuded from its host tree but may also contain modern air (Berner and Landis, 1987). Roedder (1984) also suggested that the trapped air might be mixed with volatile components of the amber.
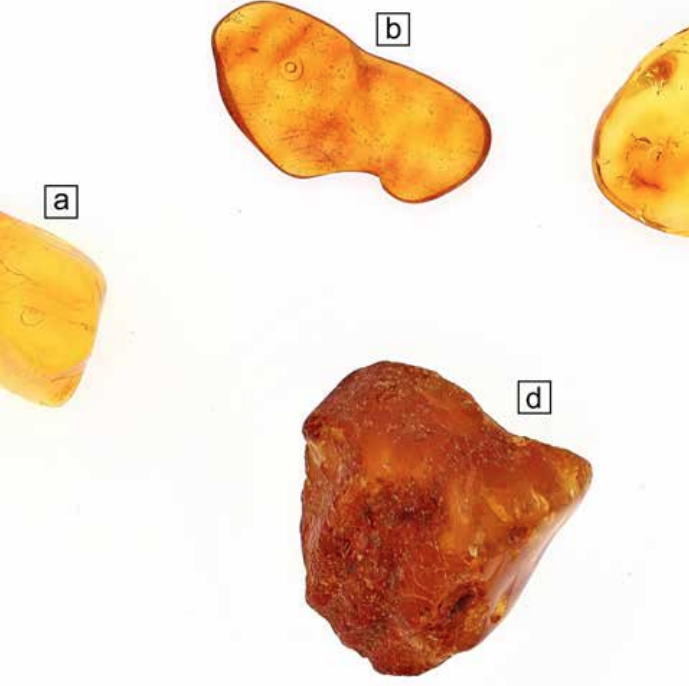
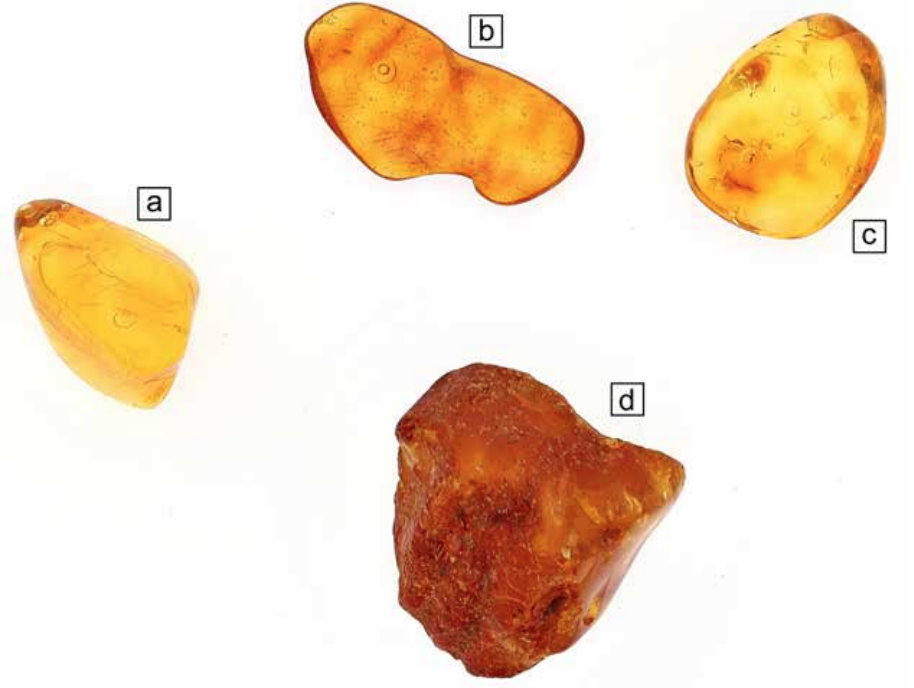
In this study, we investigate inclusions in Baltic amber, Eocene in age. Numerous samples of raw (not processed) and non-raw (processed) material are used with the aim of investigating, through a multidisciplinary approach, the nature of the inclusions and determining their origin. The samples vary in size and weight (from 0.3 to 5 grams) and have all been selected according to their inclusion’s appearance (Fig.1). The inclusions consist of air bubbles, liquid and solid phases (e.g. marcasite) as well as spores. Previous studies (Jiang et al., 2022) demonstrate that resin and amber are not always closed systems. Fluids (e.g. sediment pore water, diagenetic fluid and ground water) at different burial stages have chances to interact with amber throughout its geological history and affect the preservation quality and morphological fidelity of its organic inclusions. There are indeed numerous problems with the preservation of original compositions in the amber matrix and therefore experiments related to man-made inclusions are also carried out. The processes used to prepare the samples, such as grinding and polishing, may force the entrapment of inclusions that have nothing in common to the original entrapment, therefore, post-processing water, impurities or alteration materials need to be described and differentiated from the naturally entrapped inclusions. Data from light microscopy, scanning electron microscopy (SEM), energy-dispersive and wavelength-dispersive X-ray spectroscopy (EDX and WDX), X-ray micro-computed tomography (Micro-CT) and Raman spectroscopy will be presented.
References:
- Antoine, P.-O., De Franceschi, D., Flynn, J.J., Nel, A., Baby, P., Benammi, M., Calderón, Y., Espurt, N., Goswami, A., Salas-Gismondi, R., 2006. Amber from western Amazonia reveals Neotropical diversity during the middle Miocene. Proceedings of the National Academy of Sciences 103, 13595-13600.
- Berner, R.A., Landis, G.P., 1987. Chemical analysis of gaseous bubble inclusions in amber: the composition of ancient air? American Journal of Science, 287, 757-762.
- Berner, R.A., Landis, G.P., 1988. Gas-bubbles in fossil amber as possible indicators of the major gas-composition of ancient air. Science 239, 1406-1409.
- Briggs, D.E.G., 2003. The role of decay and mineralization in the preservation of soft-bodied fossils. Annual Review of Earth and Planetary Sciences, 31, 275-301.
- Briggs, D.E.G., 2018. Sampling the insects of the amber forest. Proceedings of the National Academy of Sciences of the United States of America, 115, 6525-6527.
- Cano, R.J., Borucki, M.K., Higbyschweitzer, M., Poinar, H.N., Poinar, G.O., Pollard, K.J., 1994. Bacillus DNA in fossil bees — an ancient symbiosis. Applied and Environmental Microbiology 60, 2164-2167.
- Cerling, T.E., 1989. Does the gas content of amber reveal the composition of paleoatmospheres. Nature 339, 695-696.
- Girard, V., Schmidt, A.R., Struwe, S., Perrichot, V., Breton, G., Néraudeau, D., 2009. Taphonomy and palaeoecology of mid-Cretaceous amber-preserved microorganisms from southwestern France. Geodiversitas 31, 153-162.
- Greenblatt, C., Davis, A., Clement, B., Kitts, C.L., Cox, T., Cano, R.J., 1999. Diversity of Microorganisms Isolated from Amber. Microbial Ecology 38, 58–68.
- Jiang, H., Tomaschek, F., Muscente, A.D., Niu, C., Nyunt, T.T., Fang, Y., Schmidt, U., Chen, J., Lönartz, M., Mähler, B., Wappler, T., Jarzembowski, E.A., Szwedo, J., Zhang, H., Rust, J., Wang, B., 2022. Widespread mineralization of soft-bodied insects in Cretaceous amber. Geobiology, 20, 363-376.
- Roedder, E., 1984. Fluid Inclusions. Reviews in Mineralogy, Vol. 12, Mineralogical Society of America, 644 p.
- Ross, A.J., 2021. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2020. Palaeoentomology, 4(1), 57-76.
- Sadowski, E.-M., Schmidt, A.R., Seyfullah, L.J., SolórzanoKraemer, M.M., Neumann, C., Perrichot, V., Hamann, C., Milke, R., Nascimbene, P.C., 2021. Conservation, preparation and imaging of diverse ambers and their inclusions. Earth-Science Reviews, 220, 103653.
- Schmidt, A.R., Ragazzi, E., Coppellotti, O., Roghi, G., 2006. A microworld in Triassic amber. Nature 444, 835.
- Schmidt, A.R., Perrichot, V., Svojtka, M., Anderson, K.B., Belete, K.H., Bussert, R., Dörfelt, H., Jancke, S., Mohr, B., Mohrmann, E., Nascimbene, P.C., Nel, A., Nel, P., Ragazzi, E., Roghi, G., Saupe, E.E., Schmidt, K., Schneider, H., Selden, P.A., Vávra, N., 2010. Cretaceous African life captured in amber. Proceedings of the National Academy of Sciences. 107, 7329-7334.
- Seyfullah, L.J., & Schmidt, A.R., 2015. Fossil focus: Stuck in time–life trapped in amber. Palaeontology, 5(12), 1-11.